Abstract
Background: Venous thromboembolism (VTE) in critically ill children is associated with increased morbidity and mortality. Therefore, significant efforts to identify children with a predisposition to VTE are being made so that prevention strategies can be implemented. To identify these children a priori, our group developed the Peds Clot Clinical Decision Rule based on exposure to known VTE risk factors (Sharathkumar et al Journal of Thrombosis and Haemostasis, 10: 1326-1334). Since this decision rule was developed using a retrospective case-control study design, we performed prospective validation of the PCDR utilizing the automatic data-collection tool available via our institution's Computer Data Warehouse (CDW). The interim analysis( Prospective Validation of the Peds Clot Clinical Decision Rule [Pdcr] in Hospital-Acquired Venous Thromboembolism: An Interim Analysis Blood 2016 128:3812) showed that three of the six variables, immobility, blood stream infection (BSI), and oral contraceptive pill (OCP) use, required manual entry. To capture immobility objectively, we took advantage of a recent initiative to record immobility of patients 13 and older at Lurie Children's Hospital. This report summarizes the analysis of this ongoing validation effort.
Objective: 1. To validate the Peds Clot Clinical Decision Rule [PCDR] utilizing automatic data collection at Lurie Children's hospital; 2. To evaluate whether the sensitivity and specificity of PDCR improves after objective assessment of immobility.
Methods: The Computer data warehouse (CDW) facility was used to directly import relevant variables into the database for our study period of 03/01/16-03/31/17. Inclusion criteria for the study were Intensive Care Unit (Pediatric, Cardiac, Neonatal) stay for at least 48 hours or more. The following demographic and clinical variables were included in the dataset: age, sex, ethnicity, date of admission and discharge, central venous catheter (CVC), BSI, immobility (defined as being incapable of ambulating without assistance), OCP use, mechanical ventilation, and length of hospitalization (LOH). Risk factors were scored as per PCDR rule1. Chi-square tests were used to examine the association between the potential risk factors and VTE. Risk factors with an association to VTE in univariate analysis were included in multivariate logistic regression model. PCDR model performance was evaluated by reporting the sensitivity and specificity. Additional independent multivariate analysis was performed to explore the role of other variables in the development of VTE.
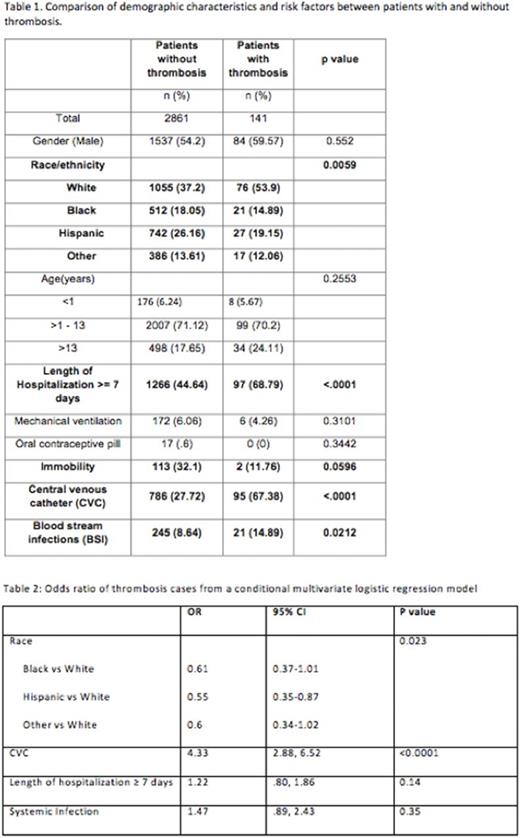
Results: A total of 2836 children were eligible for analysis. The demographic and clinical features are detailed in Table 1. Of 2836 patients, 141 (4.9%) were identified as having VTE and 881 (31%) had a CVC. LOH, presence of CVC, and BSI continued to be significantly associated with a VTE event. There was an association between VTE and immobility, but it did not reach statistical significance. The model performance showed that at the cutoff point of 3, the specificity and sensitivity of the PDCR in predicting VTE is 62% and 70% respectively. This is an improvement from our interim analysis report which had a specificity of 75% but a very low sensitivity (37%). Presence of a CVC was independently associated with VTE risk (AOR=4.33; 95% CI=2.88, 6.52). Caucasian race emerged to be associated with VTE and but did not retain its significance in multivariate analysis. Interestingly, other variables (immobility, length of stay, OCP) did not show an association with VTE in the multivariable analysis.
Conclusions: Our study confirms that automatic data collection is feasible with electronic medical records and data-ware house facility. Diligent efforts to collect standardized data for immobility resulted in only a minor improvement in the performance of the PCDR. This suggests that unlike in the general hospital population, underlying disease processes may be more important determinants of a procoagulant phenotype in critically ill patients. We plan to assess these processes in our next analysis. It is noteworthy that prospective data-collection improved the sensitivity of PCDR. This will help clinicians closely monitor this population and reduce or avoid exposure to risk factors for VTE.
Sharathkumar: Shire: Consultancy; CSL Behring: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal